EUROPE
CE Mark Certification for Medical Devices
A medical device can only be sold in Europe with a CE Mark. By placing the CE mark on a product, the manufacturer declares that its product complies with all applicable European Medical Device Regulations. As a full scope Medical Device Consultancy, we can support CE certification for your Class I, Class IIa, Class IIb or Class III devices.
EU-MDR AND EU-IVDR
Medical Device Regulation and IVD Regulation
The final texts of the new European Medical Devices Regulation (MDR) and IVD Regulation (IVDR) have been published in the Official Journal of the European Union. Entry into force commenced on May 25th 2017, marking the start of the transition period for manufacturers selling medical devices into Europe. The MDR, which replaces the Medical Devices Directive (93/42/EEC) and Active Implantable Medical Devices Directive (90/385/EEC), has a transition period of three years. The IVDR, which replaces the IVD Directive (98/79/EC), has a transition period of five years. Manufacturers have the duration of the transition period to update their technical documentation and processes to meet the new requirements.
EU-MDR CHECK
Did you check your EU-MDR compliance?
Or do you want to know all these impacts on your company? We provide a free compliance check.
EU-MDR Compliance Check
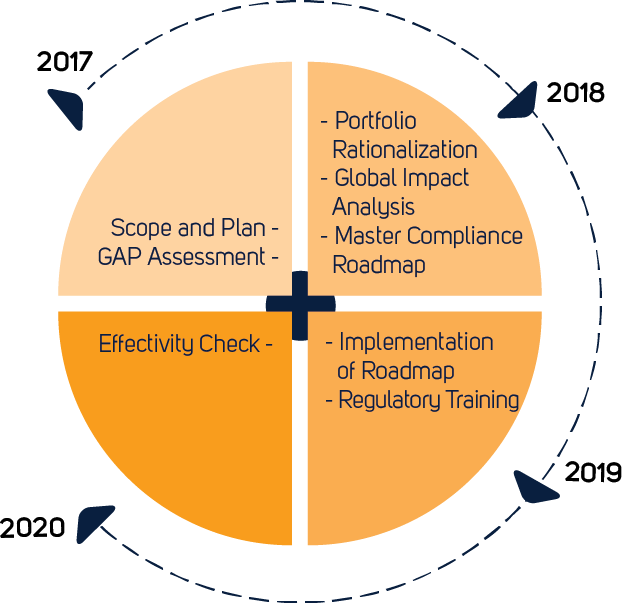
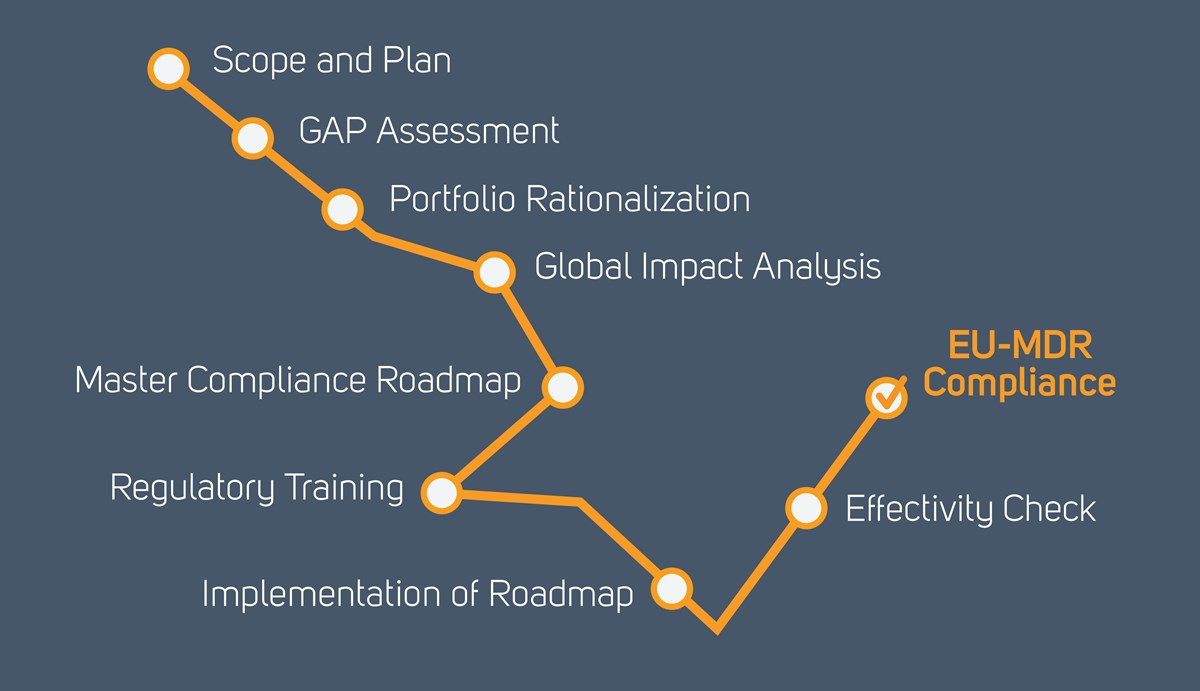
ROADMAP
The Qserve roadmap to EU-MDR Implementation
All manufacturers need to start considering the impact of the EU MDR on their activities, and what they need to do to be compliant with the revised requirements. Implementing the MDR requires a structural approach since the transition to the new MDR CE certificates can last over several years. The Qserve EU-MDR roadmap can be tailored to the specific needs of manufacturers.