Mergers & Acquisitions
Regulatory due diligence during mergers and acquisitions.
We address regulatory risks
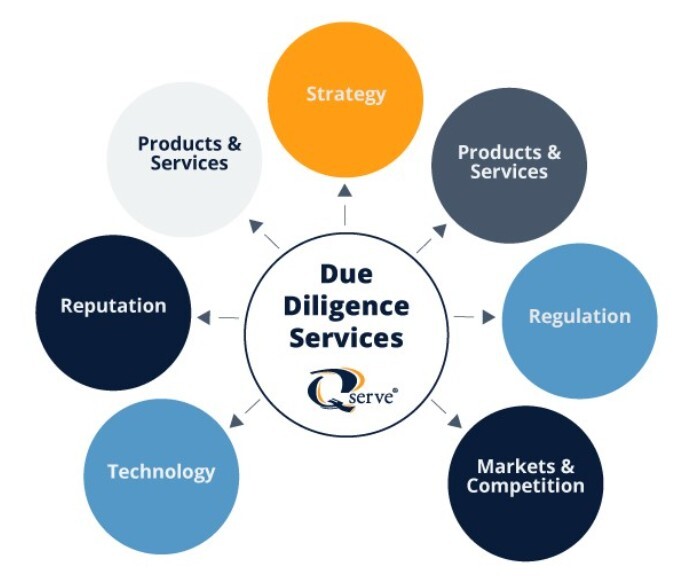
Ongoing changes in FDA regulations, along with compliance requirements under the EU MDR and IVDR, are increasingly influencing deal structures and valuations in the MedTech sector. As regulatory scrutiny intensifies and frameworks grow more complex across global markets, thorough regulatory compliance due diligence has become a critical factor in mergers and acquisitions. Ensuring alignment with evolving standards is essential for mitigating risk and securing long-term value in today's dynamic investment landscape.
At Qserve, we understand the high stakes in merger and acquisition processes. Our team of principal consultants assesses the regulatory compliance level of your target company, identifying potential regulatory risks before they become costly liabilities post-acquisition. During the due diligence period, we review the compliance level of the product portfolio and the robustness of the target company's QMS. We address potential regulatory risks related to products and markets.
Satisfied Clients
